
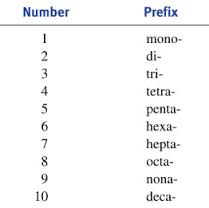
Rule #4 When necessary use the lowest number to give the location of each side chain. WAIT.these are different structures with the same names. Rule #3- Multiple side chains will use prefixes 2 is di-, 3 is tri-, 4 is tetra- and so on.Ģ separate, one carbon side chains is dimethyl The longest chain is 3 carbons, so "propane".
They are placed before the longest chain when naming. Rule #2- Remaining side chains will be given the ending -yl. Rule #1- Name the longest continuous chain of carbon atoms, and end it with -ane. Naming Compounds- Ignore all the hydrogen's. Makes for a good multiple choice question. The first 3 alkanes have no isomers (they can only be drawn 1 way). So let's look at the structural formulas and name each. You never really know how the molecule is constructed. Homologous Series- Did you notice that as you go down from CH 4 to C 2H 6 (and so on) the next member is different by 1 carbon and 2 hydrogen?
The general formula is C nH 2n+2, n is the number of carbons is used to determine the number of hydrogen atoms. The rule for naming is they all end with "-ane". I am going to take you through all the isomers of methane through octane.Īlkanes- Are saturated (all single bonds) hydrocarbons (hydrogen and carbon only). If you just want the rules click here=> RULES. I found over the years that just giving the rules is overwhelming for naming organic compounds.


 0 kommentar(er)
0 kommentar(er)
